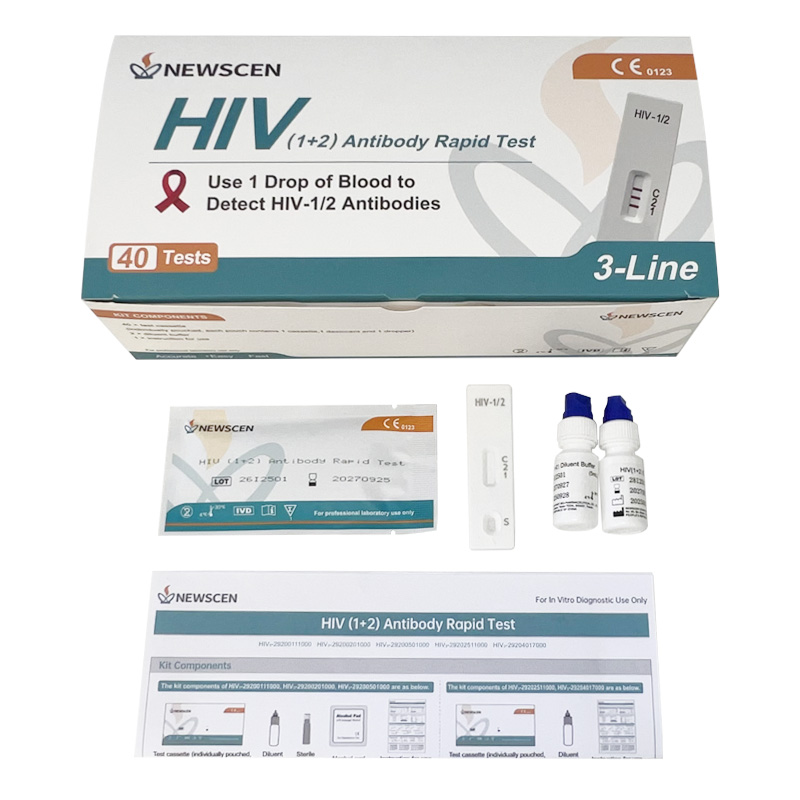
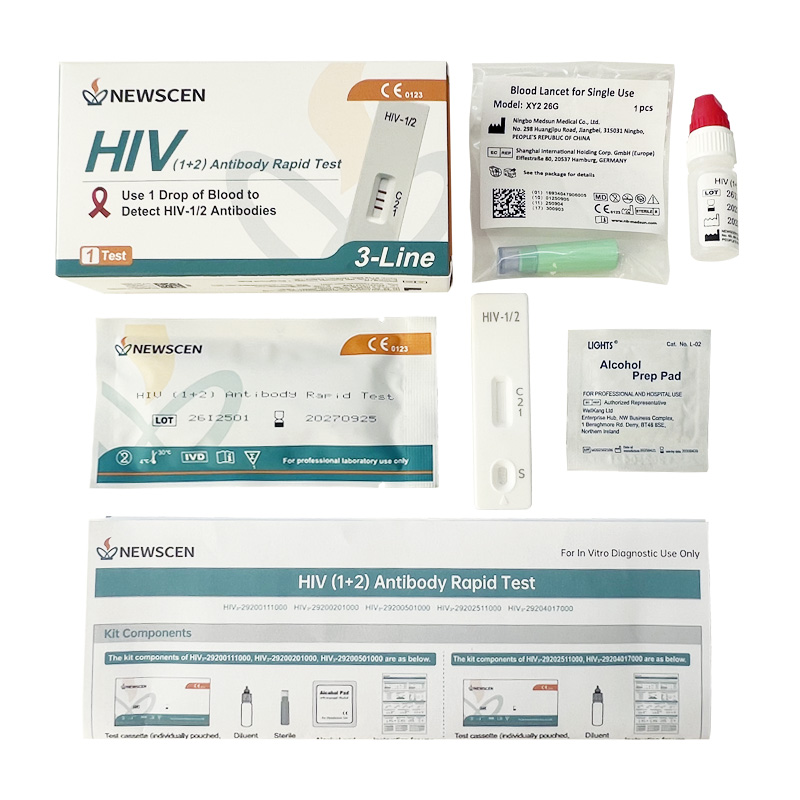
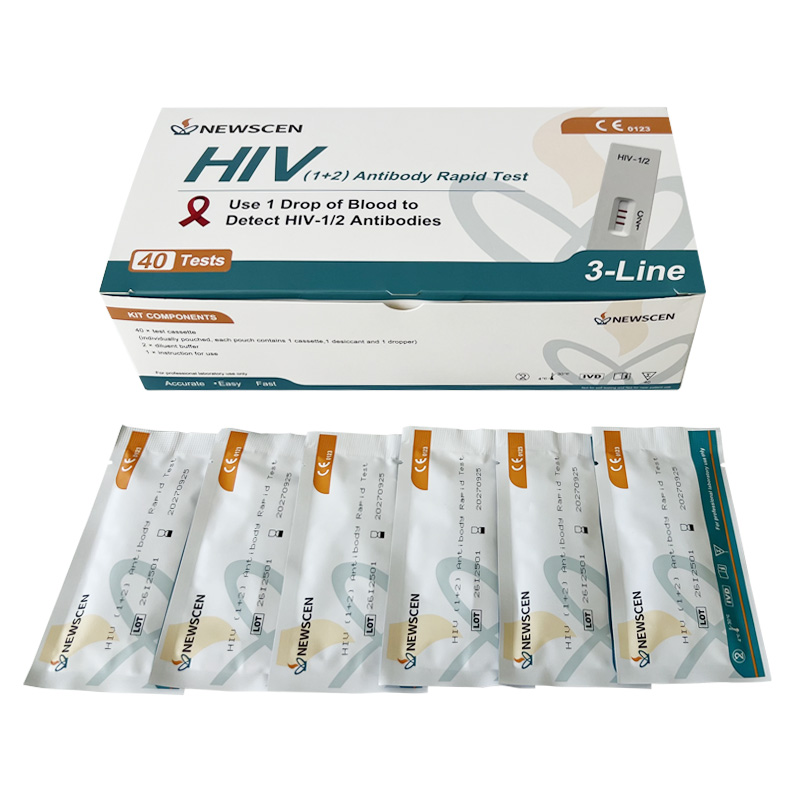
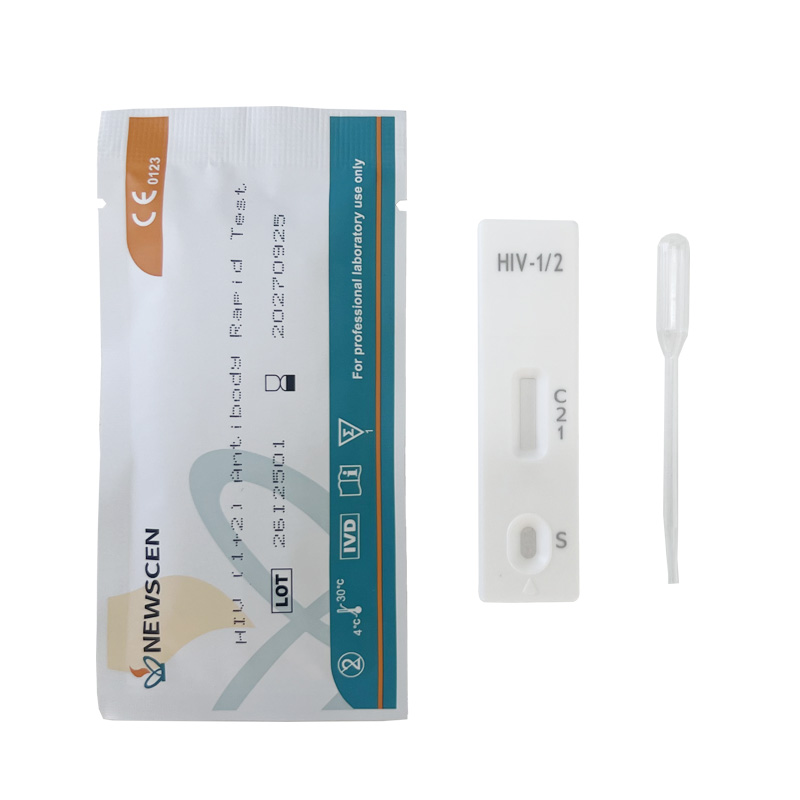
Key Words: NewScen HIV (1+2) Tri-Line Rapid Test, CE IVDR Class D HIV Rapid Test Kit, HIV Rapid Test Kit Manufacturer China
Sample type: Serum, Plasma, or Whole Blood
Detection type method: Qualitative Colloidal Gold
Usage/Application: For professional laboratory use only
Format: Cassette
Certificate: CE (IVDR Class D, EU) / NMPA (China) / ISO 13485
Sensitivity: 100%
Specificity: 99.82%
Reading time: 15-30 minutes
Packaging Details: 1 / 2 / 5 / 25 / 40 Tests per Kit (customizable)
Description
1. Product Advantages:
-
CE IVDR Class D certified by TÜV SÜD — highest regulatory standard for HIV rapid tests.
-
IVDR clinical evaluation: 100% sensitivity, 99.8% specificity
WHO evaluation report: 100% sensitivity, 100% specificity -
Tri-line differentiation design distinguishes HIV-1, HIV-2, and dual infections.
-
Early detection — detects infection as early as 7 days, ahead of most competitors
-
Room-temperature storage (4–30 °C), no instrument required — easy to use and ideal for field screening.
-
Multiple formats (1 / 2 / 5 / 25 / 40 tests) available for professional use.
-
ISO 13485 & NMPA certified manufacturing quality.
-
Proven performance in CDC and government screening programs.
2.For detailed test procedure, interpretation, and precautions, please refer to the Instructions for Use