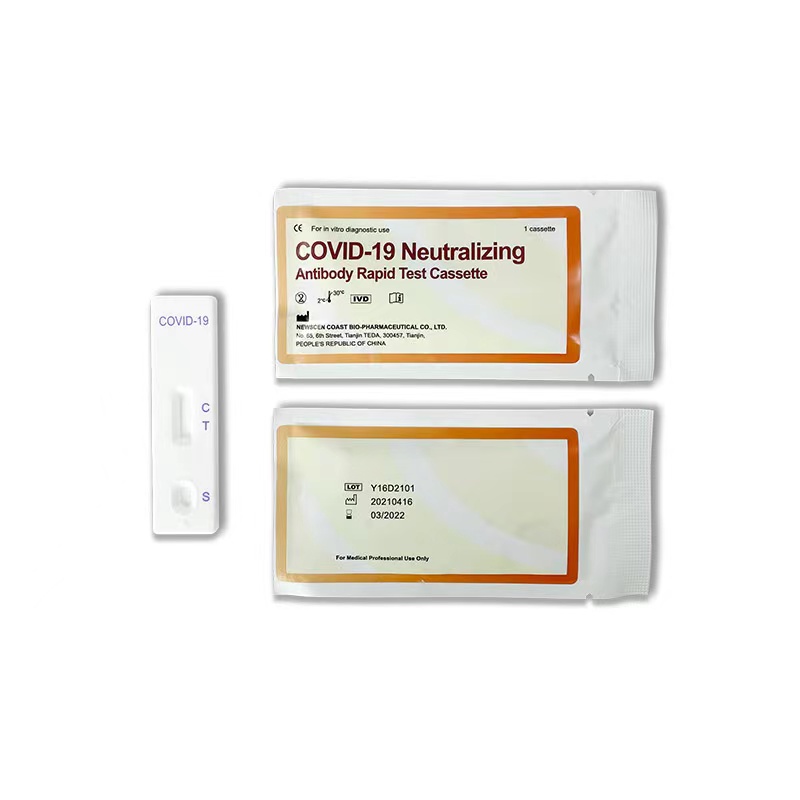
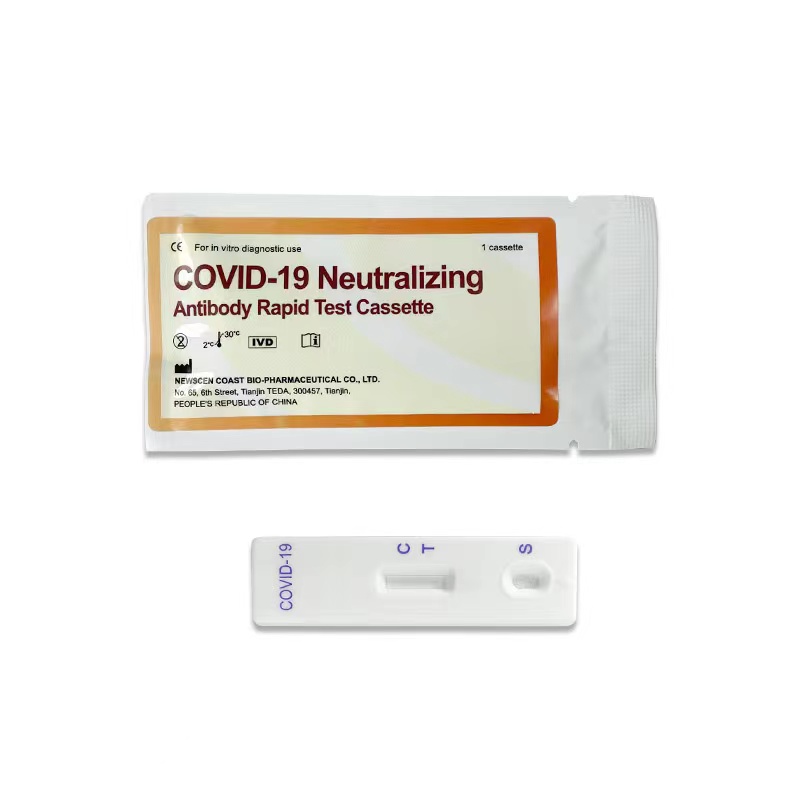
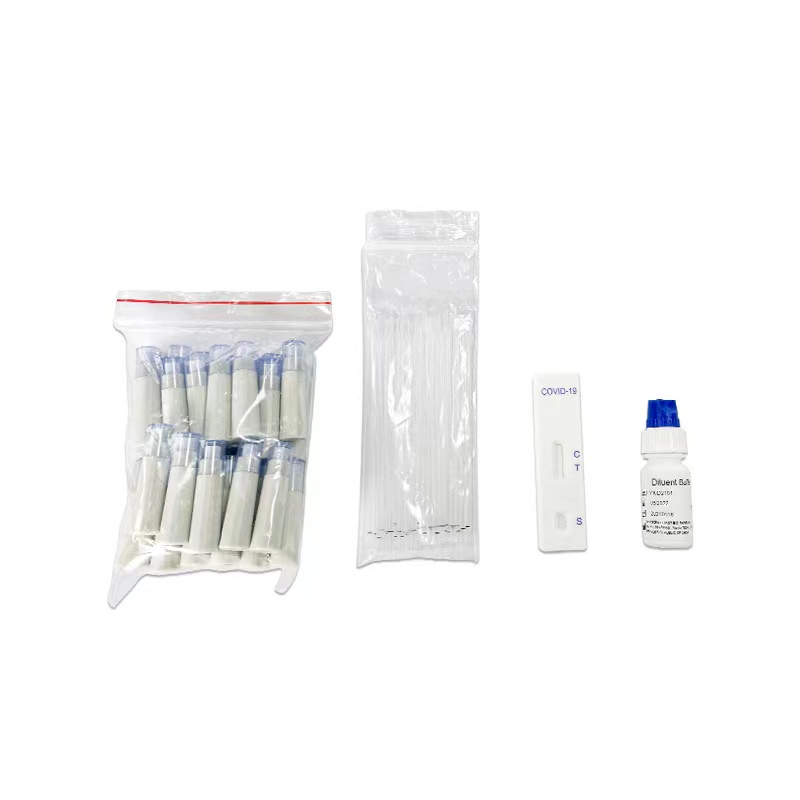
Key Words: NewScen COVID-19 Neutralizing Antibody Rapid Test Cassette For the qualitative detection of SARS-CoV-2 neutralizing antibodies in serum/ plasma and whole blood
Sample type:Blood/Serum/Plasma
Detection type:Qualitative
Method: Colloidal Gold Rapid Test
Function: Diagnose
Certificate: ISO9001/ ISO13485/CE
Format: Strip, Cassette, Midstream
Sensitivity:99.04%
Specificity: 97.56%
Accuracy: 98.62%
Reading time: 10-20 minutes
Packaging Details:
Pouch+Box+Carton packaging
(1) With our company’s Logo
(2) With the natural package
(3) With OEM package
(4) ODM
Description
1.Product Description:
The COVID-19 Neutralizing Antibody Rapid Test Cassette is for the detection of SARS-COV-2 neutralizing antibodies (NAb), which can be used to determine the immunity status after infection or vaccination.
Similar to many infectious diseases, neutralizing antibodies can help to inhibit SARS-CoV-2 replication, which means the level of neutralizing antibodies correlates to the immunity of future SARS-CoV-2 infections. Rapid detection of neutralizing antibodies can help with vaccine development, plasma therapy, and immunology study.
2.Rapid detection of neutralizing antibodies would contribute to aspects below :
*To evaluate the effectiveness of the SARS-CoV-2 vaccine in individuals
*Screening of repurposed drugs for COVID-19
*Establishment of dynamic monitoring mechanism of herd immunity
*Assistance in epidemiological investigation
3.Features :
- Proved by clinical performance with subjects from convalescent patients, vaccinated individuals, and healthy unvaccinated individuals
- Compatible with S/P/WB ( fingertip / veinpuncture )
- Easy-to-use, prompt diagnosis within 15 minutes
- Room temperature storage and transportation
- Available in 1 pcs/box, 40 pcs/box
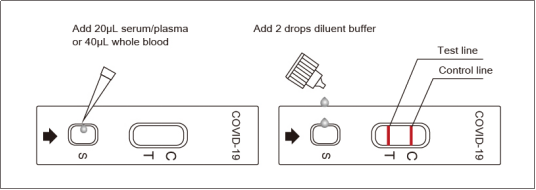
4.Operation Procedure of COVID-19 Neutralizing Antibody Rapid Test:
Step 1:
Disinfect the area to be lanced with an alcohol swab, squeeze the end of the fingertip and pierce it with a sterile lancet, wipe away the first drop of blood, collect from the second drop.
Obtain 40μL whole blood with the plastic dropper.
Step 2:
Add 40μL of whole blood, (OR 20μL serum/plasma) sample, into the“S” well.
Step 3:
Add 2 drops of the diluent buffer into the “S” well.
Step 4:
Start the timer, test result visually observed in 15 min.
NOTE:
All clinical samples must be at room temperature before beginning the assay. The test result is VALID within 20 min.
5.Result Interpretation:
5.1.Positive: One color line in the control zone (C) and one color line in the test zone (T). This indicates that the sample contains SARS-CoV-2 neutralizing antibodies.
5.2.Negative: Only one color line in the control zone (C). indicates that no SARS-CoV-2 neutralizing antibodies have been detected.
5.3.Invalid: If no color line appears in the control zone (C), whether or not a color line appeared in the test zone (T), all showed that the test is invalid. Discard the test cassette and perform with a new cassette.
Remark:
Positive (+) BOTH C & T Lines Appear
Negative (-) ONLY C Line Appears
Invalid (×) NO C line Appears
6.Sensitivity and Specificity:
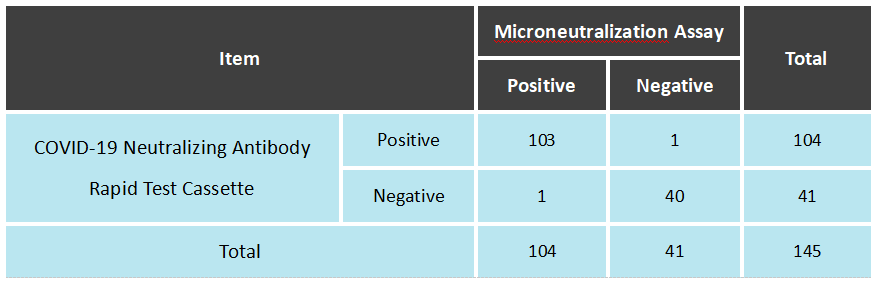
Clinical Performance using Microneutralization Assay as the comparator method with samples from convalescent patients vaccinated individuals, and healthy unvaccinated individual:
A total of 145 samples collected from convalescent patients, vaccinated individuals (Inactivated SARS-CoV-2 Vaccine), and healthy unvaccinated individuals (104 positive and 41 negatives) were evaluated the COVID-19 Neutralizing Antibody Rapid Test Cassette.
Relative Sensitivity : 103 / (1+103) = 99.04% (95% CI : 94.76%~99.98%)
Relative Specificity : 40 / (40+1) = 97.56% (95% CI : 87.14%~99.94%)
Accuracy : (103+40) / (103+1+1+40) = 98.62% (95% CI : 95.11%~99.83%)
* CI means confidence interval
7.CE Qualified:
8.What are Neutralizing Antibodies?
Not all the antibodies are neutralizing. Non-neutralizing antibodies, or binding antibodies, are able to bind to viral antigens but do not block viral infection. Binding antibodies can flag the viral antigen to trigger immune responses but the presence of binding antibodies does not reflect the level of immunity. Neutralizing antibodies (NAbs) are antibodies that not only bind to viral antigens but also block viral infection. The presence of NAb can be used to evaluate immunity status after infection or vaccination.
9.Neutralizing Antibody Rapid Test Intended Use:
The 2019 novel coronavirus (SARS-CoV-2) has several structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N). The S-protein contains a receptor-binding domain (RBD), which can recognize the cell surface receptor, angiotensin-converting enzyme-2 (ACE2). In a recent study, neutralizing antibodies (NAb) can block the interaction between the receptor-binding domain (RBD) of the novel coronavirus spike protein with the ACE2 cell surface receptor. The level of NAb, therefore, can be used to analyze a patient’s immunity against future SARS-CoV-2 infection. This COVID-19 neutralizing antibody lateral flow assay rapidly detects any antibodies that can neutralize the RBD-ACE2 interaction.
Researchers have been using traditional viral neutralization assay for testing SARS-CoV-2 neutralizing antibodies and there are several ELISA-based neutralizing antibody test kits available in the market since May 2020. However, performing COVID-19 neutralization assays or ELISA requires complex laboratory settings, and it is time-consuming, despite a higher sensitivity and specificity. NewScen’s surrogate neutralizing antibody rapid test cassette provides an easy way for the preliminary screening of NAb to estimate patients’ immunity to novel coronavirus infection.
10.How many neutralizing antibodies are necessary to prevent coronavirus? (Answer by nature medicine)
Please see the Resources section from nature medicine: