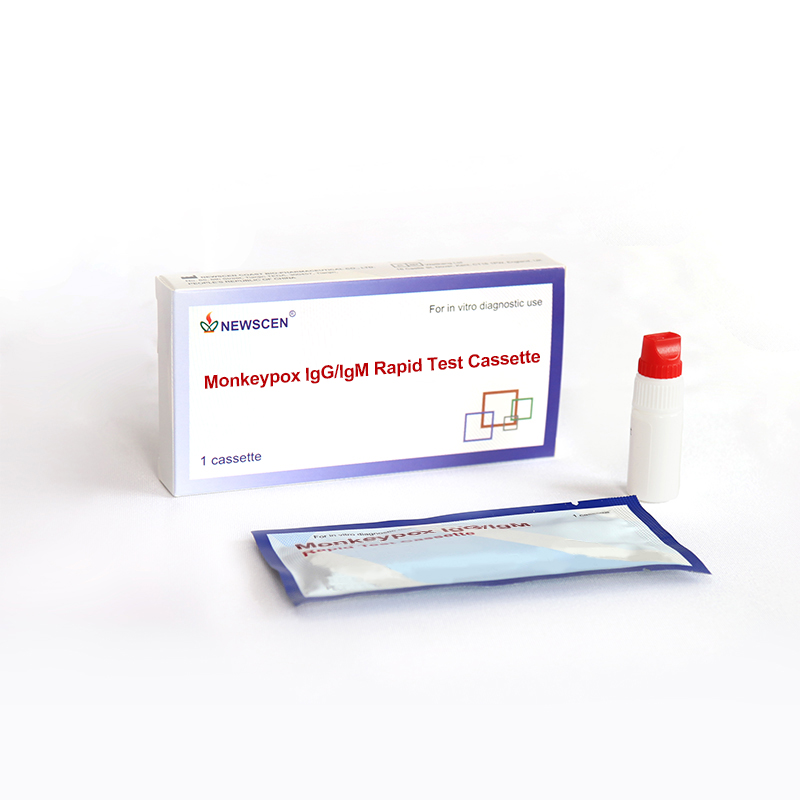
Key Words: Monkeypox Virus IgG/IgM Antibodies Rapid Test Cassette (Colloidal gold), Monkeypox Virus Test Kit, Rapid Monkeypox diagnostic test
Sample type: Serum, Plasma, or Whole Blood
Detection type&Method: Qualitative Colloidal Gold
Usage/Application: Laboratory, Hospital&Private Clinic, Pathology, Factory, School, Community Center, Self Test
Format: Cassette, Uncut sheet
Reading time: 15-30 minutes
Packaging Details:
①For Professional Use: 5 Cassette/Kit; 25 Cassette/Kit; 40 Cassette/Kit; (For Customization)…
②For Self Testing: One Cassette/Kit
③Uncut Sheet for OEM
Description
NewScen Monkeypox IgG/IgM Rapid Test Cassette is for the detection of IgG/IgM in human serum, plasma or whole blood.
1. Monkeypox IgG/IgM Rapid Test Cassette?
Once infected by monkeypox virus, people can develop monkeypox IgM and IgG antibodies in about 3-7 days.
NewScen kit uses colloidal gold immunochromatography and indirect immune capture principle of comprehensive detection technology to detect IgG/IgM of Monkeypox in human serum, plasma and whole blood.
During detection, the colloidal gold-labeled recombinant antigens bind to the Monkeypox antibodies in the sample to form immune complexes. The immune complexes move forward along the cassette by chromatography. The IgM in the immune complexes will be captured by the pre-coated anti-human IgM on the test zone, condense the color to form the reaction line M. The IgG in the immune complexes will be captured by the pre-coated anti-human IgG on the test zone, condense the color to form the reaction line G. The colloidal gold-labeled chicken IgY antibodies are combined with the pre-coated goat anti-chicken IgY antibodies on the quality control line to form the quality control line C.
2. Which conditions does Monkeypox screen for?
*For Professional Use and Self Testing
As a Top colloidal gold rapid test kit manufacturer, NewScen successfully developed rapid test kits for detecting monkeypox of IgG/IgM antibodies in serum/ plasma and whole blood
3. Monkeypox IgG/IgM Rapid Test Procedure.
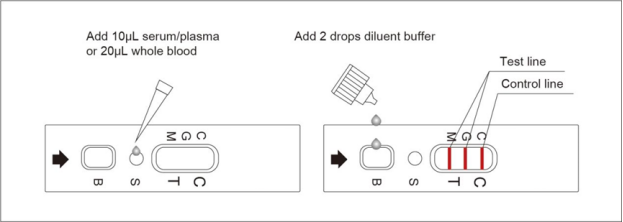
3.1. Sample preparation: Fresh serum, plasma or whole blood samples, no pretreatment is required. If the samples are stored at 2~8°C, the samples should be restored at room temperature for 15~30 minutes before use, returned to room temperature, and thoroughly mixed before testing.
3.2. Reagent preparation: Open the package, the pouch should be sealed well. If the test reagent is stored in the refrigerator, it should be restored to room temperature. Then open the packaging pouch and take out the test reagent, place it on the platform.
3.3. Detection and interpretation: Add 10μL serum/plasma or 20μL whole blood sample into S well, after the sample has permeated completely, add 2 drops of diluent buffer into B well. Read the result in 15~20 minutes, interpret the test result after 20 minutes may cause a false result.
Please contact Newscen Team to check the Instructions for Use for the complete procedure
4. Interpretation of Monkeypox IgM/IgG Test Results.
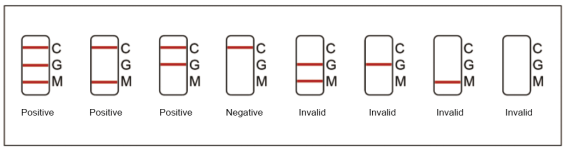
4.1 Positive IgG and IgM: One color line in the control zone (C), one color line in the test zone (G), and one color line in the test zone (M). Indicates IgG and IgM test result is positive.
4.2 Positive of IgG: One color line in the control zone (C) and one color line in the test zone (G), indicates the IgG test result is positive.
4.3 Positive IgM: One color line in the control zone (C) and one color line in the test zone (M), indicates the IgM test result is positive.
4.4 Negative of IgG and IgM: Only one color line in the control zone (C) indicates IgG and IgM test result is negative.
4.5 Invalid: If no color line appears in the control zone (C), the test is invalid. Discard the test cassette and perform with a new cassette.
4.6 Built-In Control: Monkeypox IgG/IgM Rapid Test Cassette has a built-in procedural control that demonstrates assay validity. A color line appeared on the control zone (C) indicates that the test runs correctly.
5. Become NewScen Distributor.

NewScen Coast Biotech is one of the fastest-growing manufacturers in the IVD industry, currently distributing our diagnostic test kits in over 90 countries worldwide. We are poised to rapidly expand our distribution network into new markets and increase market share in regions where we have an existing presence.
As the developer and complete manufacturer of our diagnostic test kits, we are capable of localizing products for your specific market. We are continuously developing new products and technologies in response to market demands to facilitate growth. Our distributors can also expect a high level of support through product information and training, marketing collateral, and a commitment to help them develop their business. Together, we can improve healthcare by delivering high-quality diagnostic products around the world.
Welcoming you to be our Distributor or Agent, authorizing regional protection for partners, working and developing together to bring more value for people, health business, and society, meantime, realizing your own career to be stronger and stronger.
Please contact below info with our sales team.
Web: www.newscenbiotech.com
Email: export@newscen.com