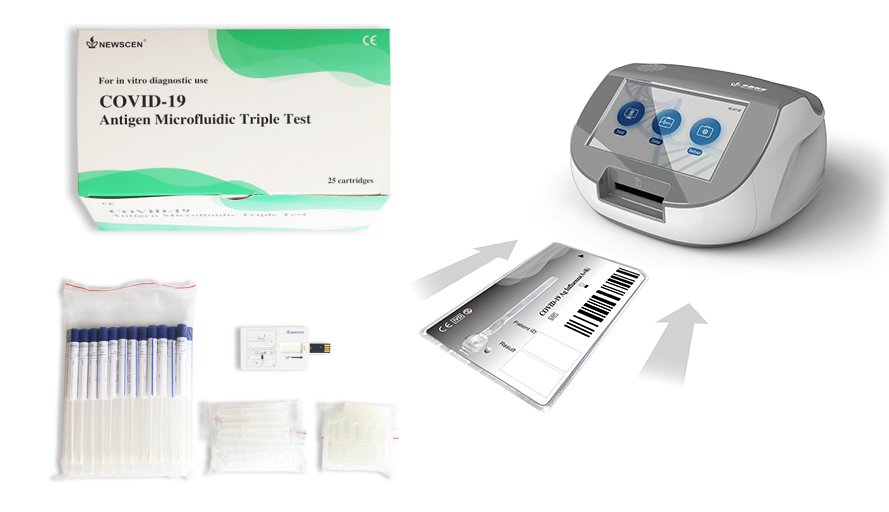
Key Words: NewScen COVID-19 Antigen Microfluidic Triple Test For the qualitative detection of antigens to SARS-CoV-2/Influenza type A/ Influenza type B in human throat swab or nasal swab.
Sample type: Throat OR nasal swab
Detection type: Qualitative
Method: Microfluidic
Usage/Application: Laboratory / Hospital / Pathology
Function: Diagnose
Certificate: ISO9001/ ISO13485/CE
Reading time: 4 minutes
Packaging Details:
Pouch+Box+Carton packaging
(1) With our company’s Logo
(2) With the natural package
(3) With OEM package
(4) ODM
Description
1. Product Description:
For the qualitative detection of antigens to SARS-CoV-2/Influenza type A/ Influenza type B in human throat swab or nasal swab.
2. Intended Use:
COVID-19 Antigen Microfluidic Triple Test is for combined in vitro qualitative detection of specific antigens to SARS-CoV-2, Influenza type A and/or type B present in the human throat or nasal cavity. It cannot be used as the basis for the diagnosis and exclusion of COVID-19. The kit is used in combination with F10Pro NewScen Fluorimetric Immunoassay Analyser produced by our factory.
This reagent is used to detect cases with suspected symptoms of COVID-19 within 7 days. If suspected symptoms are more than 7 days, it is recommended to test with COVID-19 antibodies or nucleic acid reagents. The main clinical symptoms of COVID-19 are: Fever, dry cough, fatigue, a few patients will have stuffy noses, runny nose, and diarrhea.
3. Principle:
This kit uses microfluidic technology, fluorescence technology, and double antibody sandwich technique of comprehensive detection technology to combined detect specific antigens to SARS-CoV-2, Influenza type A and type B in the human throat or nasal cavity. Microfluidic technology is a detection technology that uses the microchannel inside the reagent cartridge to realize liquid quantification and uniform flow.
During the detection, antigens of SARS-CoV-2, Influenza type A and B in the sample first react with the fluorescently labeled specific antibody to form the antigen~fluorescently labeled antibody immune complexes, the immune complexes depend on siphon action to flow forward automatically in the microchannel. When flow to the detection area, antigens of Influenza type B in the immune complexes meet the pre-coated Influenza type B paired antibodies and are absorbed in the detection area, forming the detection area T1, antigens of SARS-CoV-2 in the immune complexes meet the pre-coated SARS-CoV-2 paired antibodies and absorbed in the detection area, forming the detection area T2, antigens of Influenza type A in the immune complexes meet the pre-coated Influenza type A paired antibodies and absorbed in the detection area, forming the detection area T3. The unbound fluorescent-labeled antibodies continue to flow forward, and when they flow to the quality control area, the antibodies encounter the goat anti-mouse IgG and are absorbed in the quality control area, the residuum eventually flows to the waste area.
On NewScen Fluorimetric Immunoassay Analyser, it collects the fluorescence signal that excited by the excitation light in the detection area and the quality control area, the intensity of the fluorescence signal is positively correlated with the number of antigens to SARS-CoV-2, Influenza type A and type B present in the specimen.
4. Storage:
COVID-19 Antigen Microfluidic Triple Test should be stored in dark place at 2~8°C for 12 months from the date of manufacture. Keep the test cartridge in a sealed pouch until use. Once you have taken the test cartridge out of the pouch, use it immediately. Do not use the test beyond the indicated expiration date.
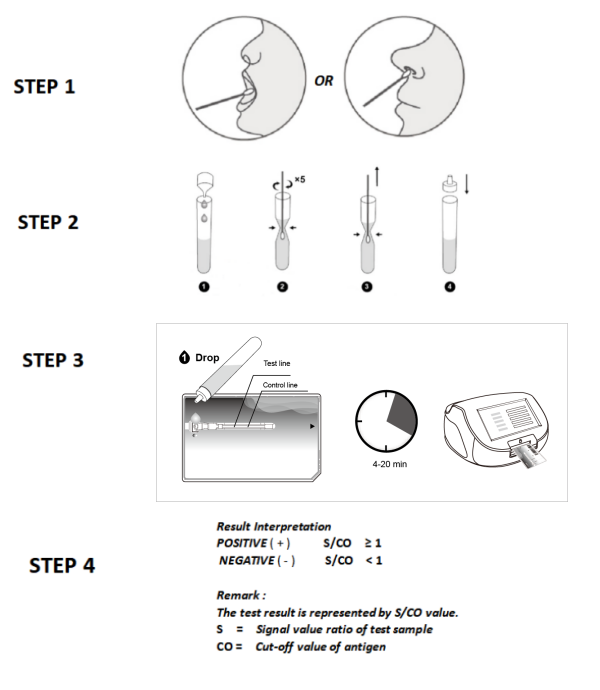
5. Procedure of NewScen COVID-19 Antigen Microfluidic Triple Test:
5.1. All clinical samples must be at room temperature before beginning the assay.
5.2. Follow the instruction and installation guide of NewScen Fluorimetric Immunoassay Analyser. Import the code chip of the kit into the instrument.
5.3. Open the package, the pouch should be sealed well. If the test reagent store in the refrigerator, it should be restored at room temperature for 15~30 minutes. Then open the pouch and take out the test cartridge, place it on the platform.
5.4. Add 1 drop of patient sample from the tube, start timing. In 4~20 minutes, insert the test cartridge in the direction of the black arrow to the instrument. The result is invalid after 20 minutes.
5.5. Before reading the reagent, confirm the instrument is in the normal operation state, and be calibrated by the instrument calibration card.
6. Result Interpretation:
The test result is represented by the S/CO value, S represents the signal value ratio of the test sample, and CO1 represents the Cut-off value of Influenza type B, CO2 represents the Cut-off value of SARS-CoV-2, CO3 represents the Cut-off value of Influenza type A.
6.1. Positive of Influenza type B: In area T1, the test result S/CO1≥1.
6.2. Negative of Influenza type B: In area T1, the test result S/CO1<1.
6.3. Positive of SARS-CoV-2: In area T2, the test result S/CO2≥1.
6.4. Negative of SARS-CoV-2: In area T2, the test result S/CO2<1.
6.5. Positive of Influenza type A: In area T3, the test result S/CO3≥1.
6.6. Negative of Influenza type A: In area T3, the test result S/CO3<1.
7. Limitation:
7.1. The kit is only used to detect human throat swabs or nasal swabs.
7.2. The accuracy of the test depends on the process of sample collection. Improper sample collection, improper sample storage, or repeated freezing and thawing of samples will affect the test results.
7.3. The test results of this reagent are for clinical reference only and should not be used as the sole basis for clinical diagnosis and treatment. The clinical management of the patient should be considered in combination with other laboratory tests of the patient’s symptoms/signs history and treatment response.
8. CE Qualified: