By 03:22 a.m., Jan. 27, 2021, Beijing time, the number of diagnosed and confirmed COVID-19 cases has exceeded 100 million globally!
As the most phenomenal and severe infectious disease known to the world in the past 100 years, the COVID-19 pandemic is rare in human history in terms of its wide range of influence, the accumulated number of fatal cases, and difficulty in fighting against.
In response to the outbreak and spread of the Novel Coronavirus Pneumonia, WHO ( World Health Organization ) has released the Instructions for Submission Requirements: In vitro diagnostics (IVDs) Detecting SARS-CoV-2 Nucleic Acid on Feb. 28, 2020. However, the application scenario of the traditional RT-PCR detection method for COVID-19 is restricted to senior CDC and CLASS III qualified hospitals or organizations. And the classical RT-PCR operation process depends on longer TAT and well-equipped large laboratories, which means the epidemic prevention and control expectation of “early detection and diagnosis” is hard to achieve.
How to accomplish the tasks of COVID-19 pandemic screening tests in low-resource settings in the “accurate, rapid, convenient and affordable” way?
Based on the Microfluidic immunofluorescence platform, the NewScen COVID-19 antigen and antibody Microfluidic RDTs series have better fulfilled the epidemic prevention and diagnosis requirements from multiple aspects :
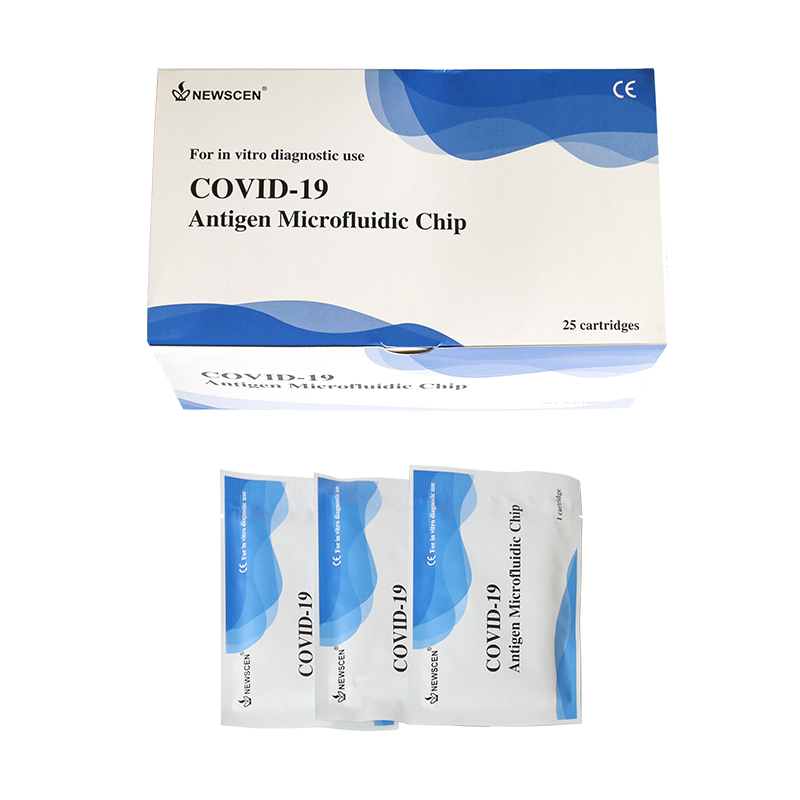
[1] COVID-19 Antigen Microfluidic Chip: Shorten the window period and Improve the detection rate for an early diagnosis of COVID-19 infection
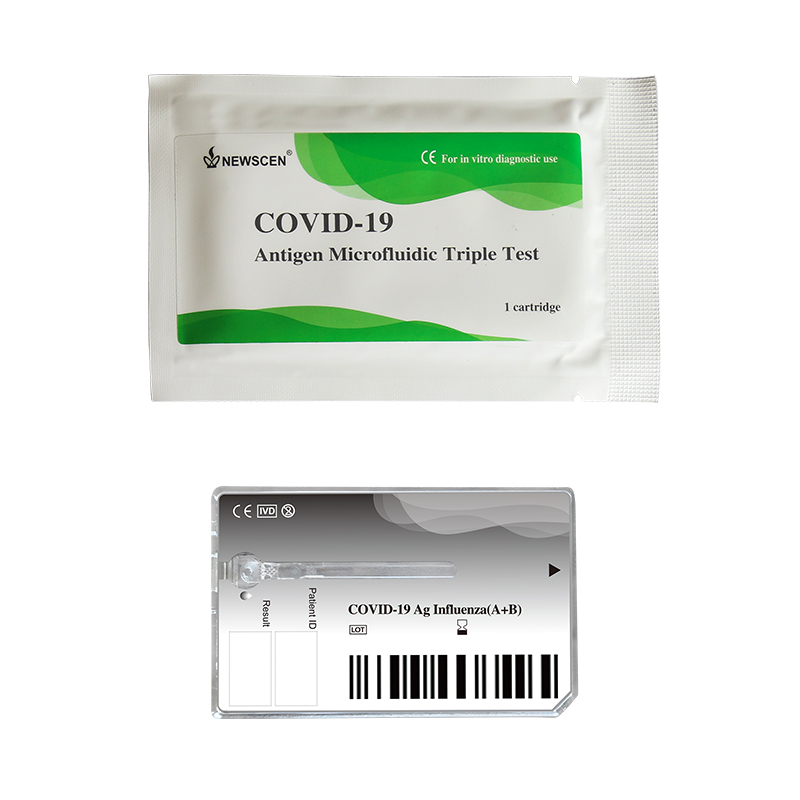
[2] COVID-19 Antigen Microfluidic Triple Test: Distinguish COVID-19 Antigen from Influenza A/B for an early precise diagnosis
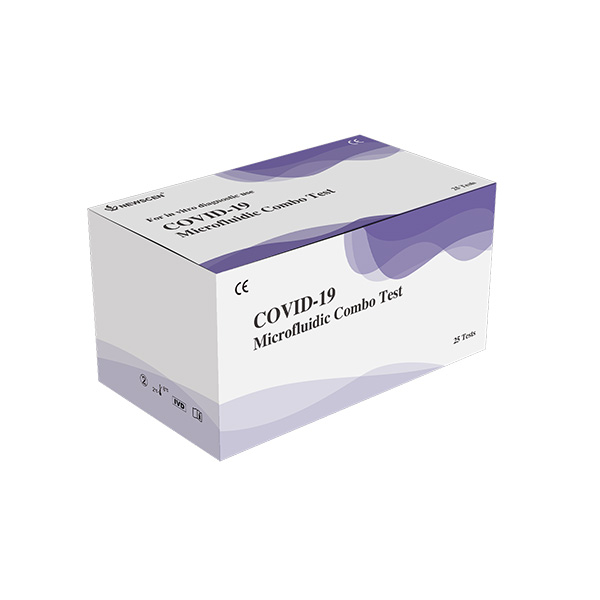
[3] COVID-19 Antigen Microfluidic Combo Test: Classify SAA/CRP for virus or bacterial infection prior to COVID-19 IgG/IgM detection
The technical advantages of the Microfluidic immunofluorescence platform have empowered NewScen to deliver “accurate, rapid, convenient and affordable” POCT products applicable for the anti-epidemic campaign and made it the optimal choice for effective implementation of COVID-19 prevention and control in public places with a high circulation of personnel including customs, airport, office building, factory, school, etc.