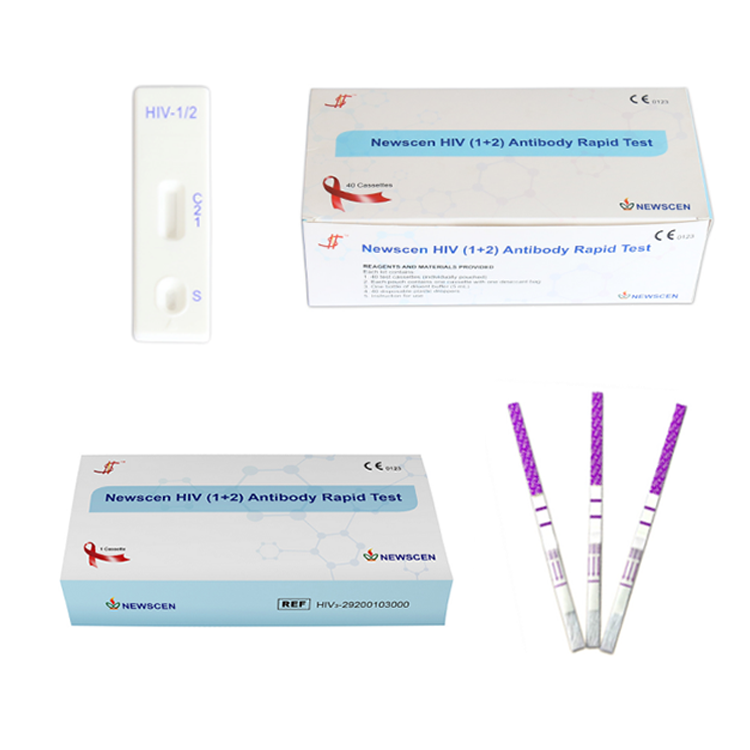
Key Words: NewScen HIV (1+2) Antibody Rapid Test, HIV 3line 1+2 Rapid Test Kit
Sample type: Serum, Plasma or Whole Blood
Detection type&method: Qualitative Colloidal Gold
Usage/Application: Laboratory, Hospital&Private Clinic, Pathology, Factory, School, Community Center, Home Self Test
CE Code: 0123
Certificate: ISO9001/ ISO13485/ CE by TUV/ MHRA/ WHO
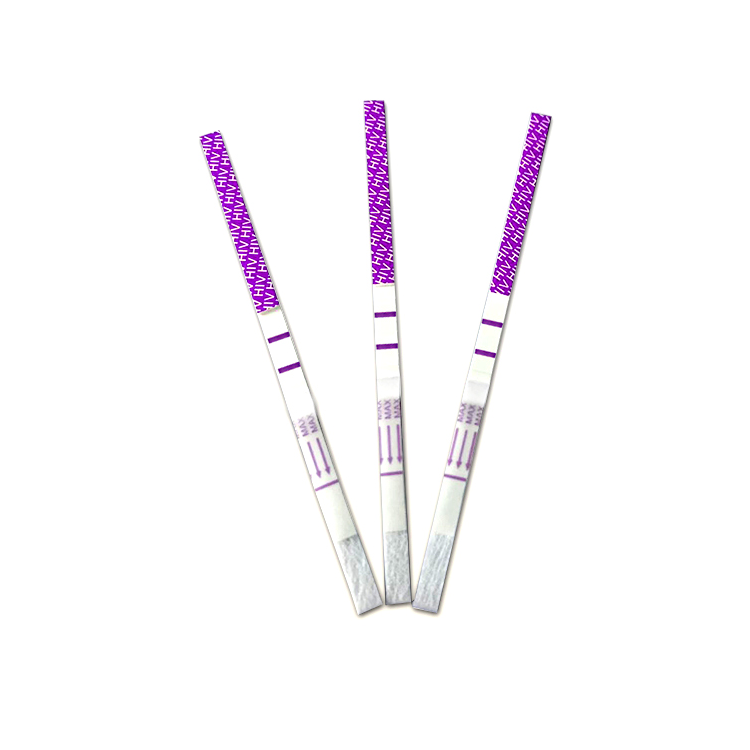
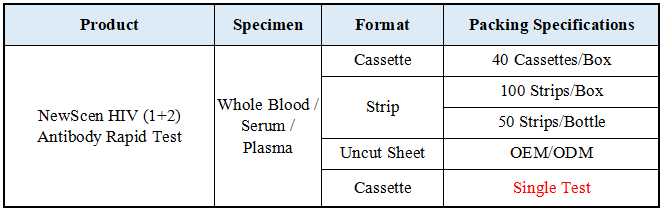
Format: Strip, Cassette, Uncut sheet
Certification Authority Achieve Zero Error
Sensitivity: 100%
Specificity: 100%
Total Accuracy: 100%
Reading time: 15-30 minutes
Packaging Details:
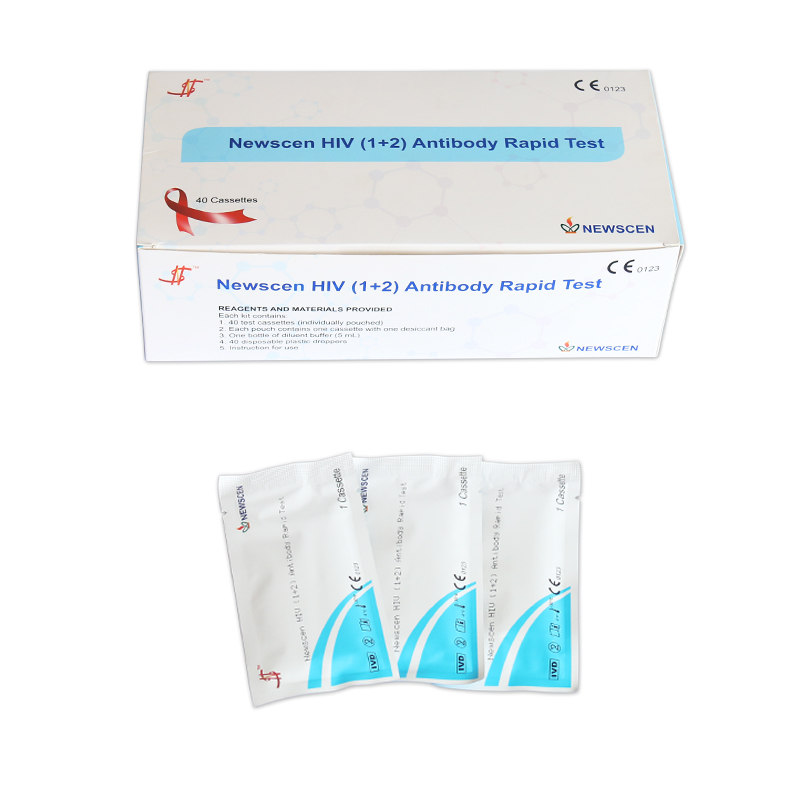
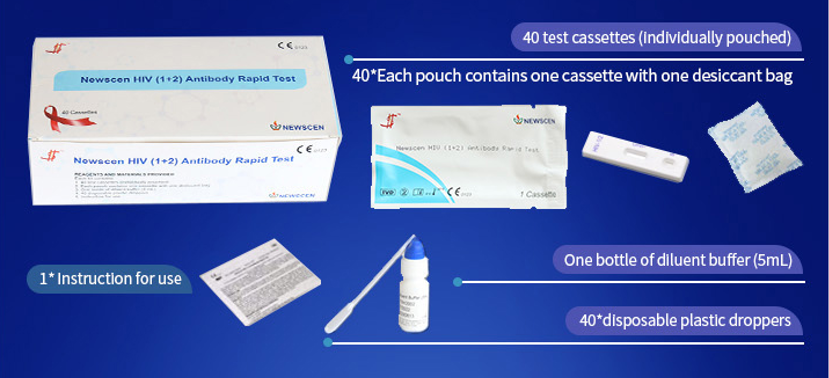
①For Professional Use: 40 Cassette/Kit; 100 Strips/Kit; 50 Strips/Bottle (For Customization)…
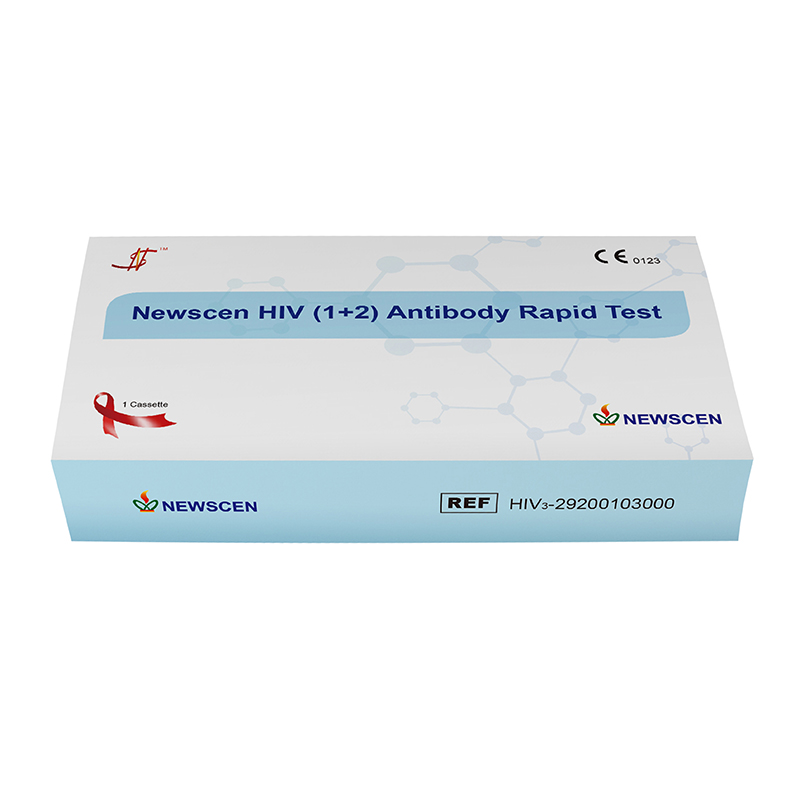
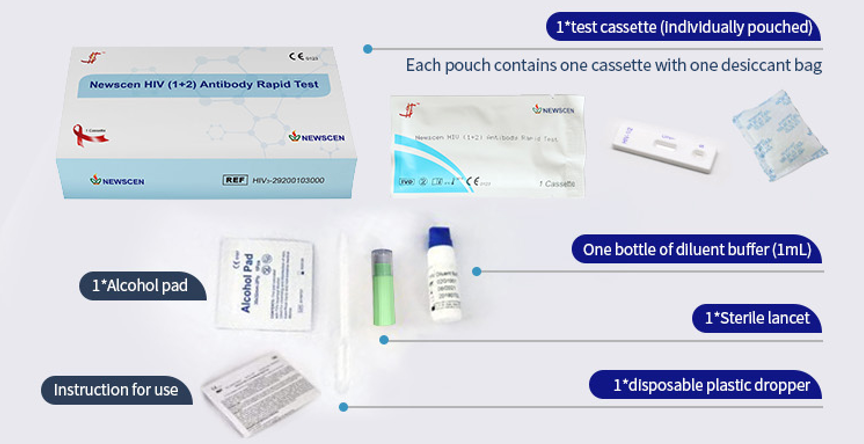
② One Cassette/Box
③Uncut Sheet for OEM
Description
NewScen is a CE, ISO, MHRA, WHO HIV Test Kit manufacturer.
One Step Anti-HIV1+2 in whole Blood Test is a rapid direct binding screening test for the presence of antibodies to HIV 1 and HIV 2. The test is based on the principle of double antigen sandwich immunoassay for the qualitative detection of Anti-HIV in human whole blood. Purified recombinant antigens are employed to identify Anti-HIV specifically. This one-step test is very sensitive and only takes about 15 minutes. Test results are read visually without any instrumentation.
1. NewScen Provide HIV Rapid Test With Below Specification.
*One Cassette, including:
*40 Cassettes/Kit for professional Use, including:
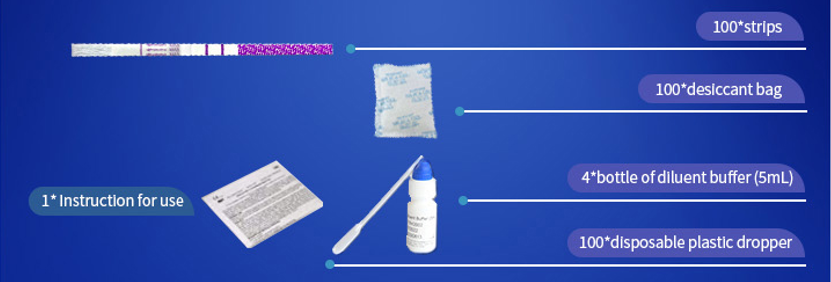
*100 strips /Box for professional Use, including:
HIV Rapid Test Uncut Sheet For Professional Use:
2. Operation of NewScen HIV Test Kit:
Click here to view the video of the operation: https://youtu.be/25_iWLp1EcE
3. International Certificates Of Newscen HIV Test Kits:
*WHO: Certification Authority Achieve Zero Error
WHO evaluation of NewScen HIV (1+2) Antibody Rapid Test: Sensitivity: 100%, Specificity: 100%, Total Accuracy: 100%
*CE (List A)
NewScen patented HIV and HCV RDTs have obtained CE approval by TUV laboratory evaluation, hot sold domestically and abroad by receiving high concentration by customers.
*MHRA
NewScen HIV (1+2) Antibody Rapid Test has been granted regulatory approval by the Medicines and Healthcare products Regulatory Agency (MHRA).