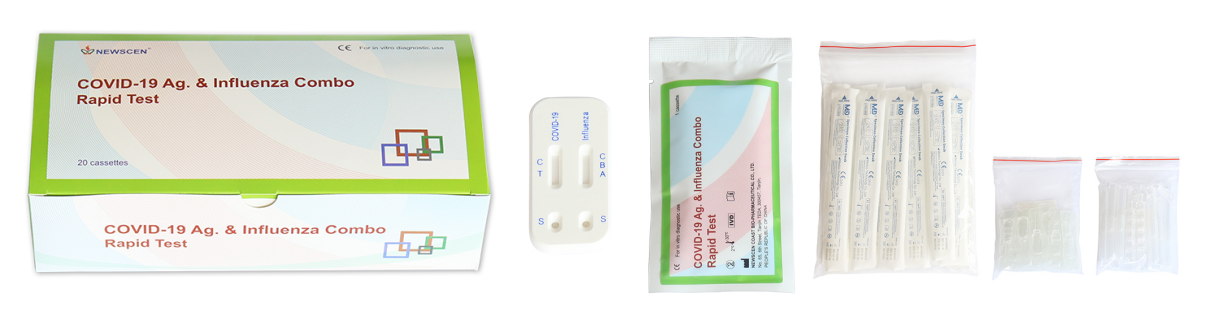
Key Words: Influenza & COVID-19 Ag Combo Rapid Test Cassette, COVID-19 & Flu Dual Antigen Test, COVID-19 Ag+Influenza A/B Test, Influenza & COVID-19 Test
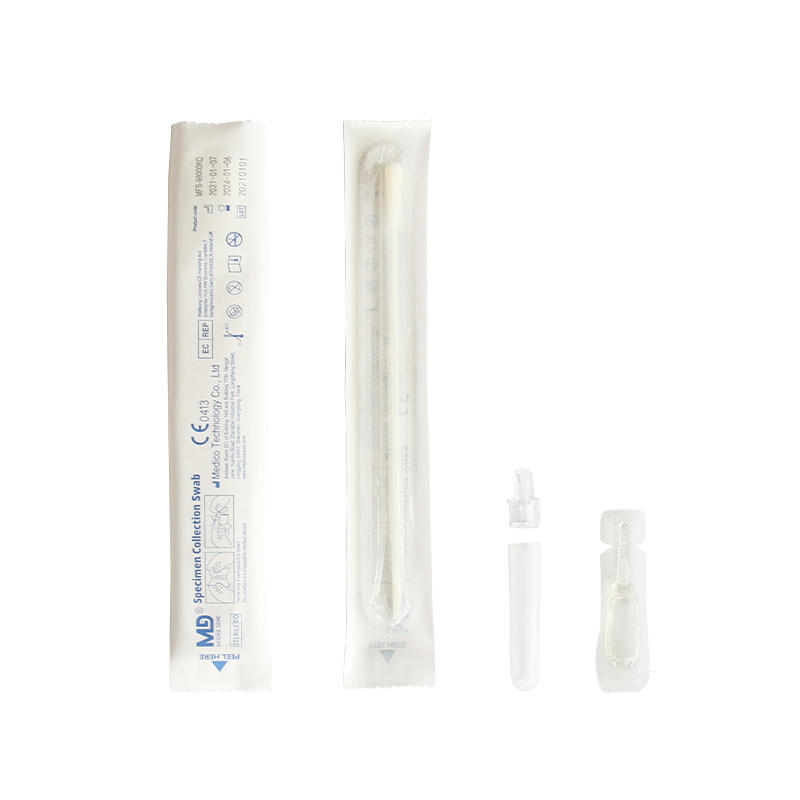
Sample type: Throat OR nasal swab
Detection type: Qualitative
Method: Colloidal Gold Rapid Test
Function: Diagnose
Certificate: ISO9001/ ISO13485/CE
Format: Strip, Cassette, Midstream
Reading time: 15 minutes
Packaging Details:
Pouch+Box+Carton packaging
(1) With our company’s Logo
(2) With the natural package
(3) With OEM package
(4) ODM
Description
1.Product Description:
COVID-19 Ag. & Influenza Combo Rapid Test is for combined in vitro qualitative detection of specific antigens to SARS-CoV-2, Influenza type A and/or type B present in human throat or nasal cavity. It is used to detect cases with suspected symptoms ( fever, dry cough, fatigue, stuffy nose, runny nose, diarrhea, etc. ) of COVID-19 within 7 days.
This reagent is CE marked and certified by China export white list.
2.Features :
- 3 parameters in1 test cassette ( Combo ) , fast & efficient
- Effective distinction between COVID-19 & Influenza A/B
- Test result visually interpreted within 15 minutes
- Room temperature storage and transportation
- Available in 20 pcs/box
3.Intended Use:
COVID-19 Ag. & Influenza Combo Rapid Test is for combined in vitro qualitative detection of specific antigens to SARS-CoV-2, Influenza type A and/or type B present in human throat or nasal cavity. It cannot be used as the basis for the diagnosis and exclusion of COVID-19.
This reagent is used to detect cases with suspected symptoms of COVID-19 within 7 days. If suspected symptoms are more than 7 days, it is recommended to test with COVID-19 antibodies or nucleic acid reagents.
The main clinical symptoms of COVID-19 are: Fever, dry cough, fatigue, a few patients will have stuffy noses, runny nose, and diarrhea.
4.Principle:
This kit uses a double-antibody sandwich immunoassay to combined detect specific antigens to SARS-CoV-2, Influenza type A and/or type B in the human throat or nasal cavity.
For COVID-19 antigen detection, the membrane was precoated with SARS-CoV-2 specific antibody on the test zone and goat anti-mouse IgG antibody on the control zone. During the test, the colloidal gold-labeled specific antibody binds to the SARS-CoV-2 in the sample to form immune complexes. The immune complexes move forward along the cassette by chromatography. The SARS-CoV-2 in the immune complexes will be captured by the pre-coated specific antibody of SARS-CoV-2 on the test zone to produce a visual red color line. Regardless of the presence of SARS-CoV-2, as the mixture continues to move across the membrane to the control zone, the complex is captured by immobilized goat anti-mouse IgG antibodies to form a distinct red line.
For Influenza detection, The Influenza type A and type B specific antibodies were separately pre-coated onto the test zone of the membrane as the test line A and test line B, and goat anti-mouse IgG antibody on the control zone. During the test, the colloidal gold-labeled specific antibodies bind to the Influenza type A and type B in the sample to form immune complexes. The immune complexes move forward along the cassette by chromatography. The Influenza type A in the immune complexes will be captured by the pre-coated specific antibody of Influenza type A on the test zone, condense the color to form the test line A. The Influenza type B in the immune complexes will be captured by the pre-coated specific antibody of Influenza type B on the test zone, condense the color to form the reaction line B. The color of the line is positively correlated with the amount of SARS-CoV-2 in the specimen. Regardless of the presence of Influenza type A and/or type B, as the mixture continues to move across the membrane to the control zone, the complex is captured by immobilized goat anti-mouse IgG antibody to form a distinct red line.
5.Storage :
COVID-19 Ag. & Influenza Combo Rapid Test should be stored in a dark place at 2~30°C for 24 months from the date of manufacture. Keep the test cassette in a sealed pouch until use. Once you have taken the test cassette out of the pouch, use it immediately. Do not use the test beyond the indicated expiration date.
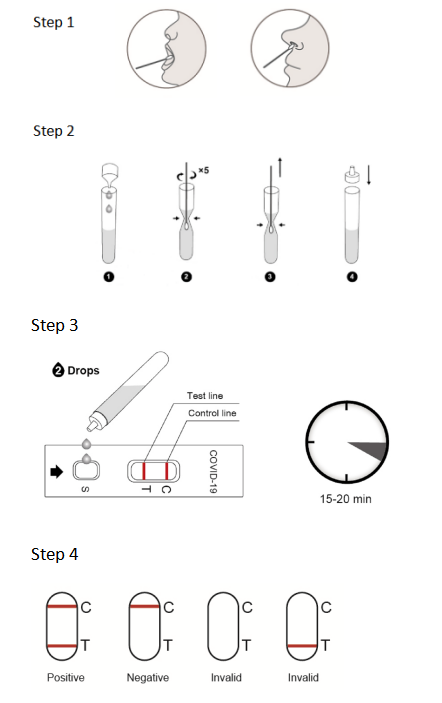
6.Procedure of NewScen COVID-19 and Influenza A+B Rapid Antigen Combo Test:
6.1. All clinical samples must be at room temperature before beginning the assay.
6.2. Open the package, the pouch should be sealed well. If the test reagent store in the refrigerator, it should be restored to room temperature. Then open the pouch and take out the test cassette, place it on the platform.
6.3. Add 2 drops of patient sample extraction solution from the tube to each S Well, Observe the result in 15~20 minutes, interpret the test result after 20 minutes may cause a false result.
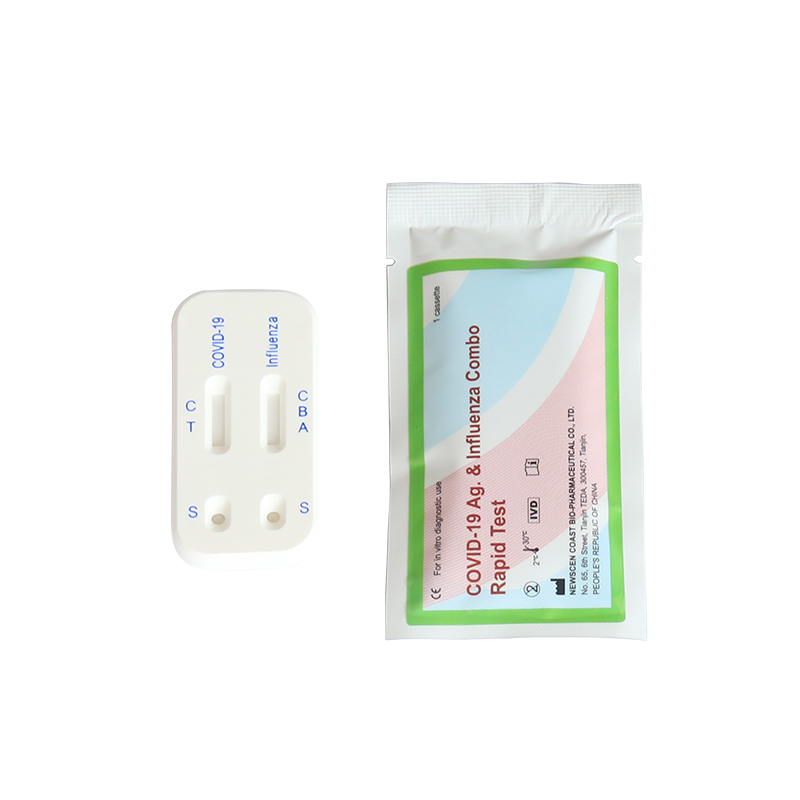
7.Result Interpretation:
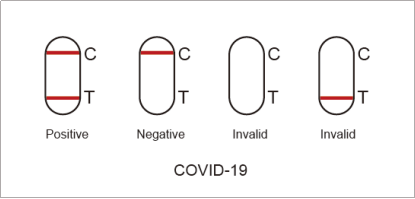
7.1. Positiveof SARS-CoV-2:One color line in the control zone (C) and one color line in the test zone (T). This indicates that the sample contains SARS-CoV-2 antigen.
7.2. Negative of SARS-CoV-2:Only one color line in the control zone (C). This indicates that no SARS-CoV-2 antigen has been detected.
7.3 Invalid of SARS-CoV-2: If no color line appears in the control zone (C), the test is invalid. Discard the test cassette and perform with a new cassette.
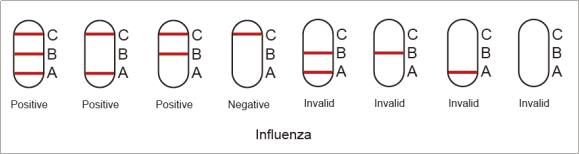
7.4. Positive of Influenza type A: One color line in the control zone (C) and one color line in the test zone (A). This indicates that the sample contains Influenza type A antigen.
7.5. Negative of Influenza type A: Only one color line in the control zone (C). This indicates that no Influenza type A antigen has been detected.
7.6. Positive of Influenza type B: One color line in the control zone (C) and one color line in the test zone (B). This indicates that the sample contains Influenza type B antigen.
7.7. Negative of Influenza type B: Only one color line in the control zone (C). This indicates that no Influenza type B antigen has been detected.
7.8. Invalid of Influenza type A and type B: If no color line appears in the control zone (C), regardless of whether there is a color line in the test zone(A and B), indicating that the test is invalid. Discard the test cassette and perform with a new cassette.
8.Limitation:
8.1 The kits only used to detect human throat swab or nasal swab.
8.2 The accuracy of the test depends on the process of sample collection. Improper sample collection, improper sample storage, or repeated freezing and thawing of samples will affect the test results.
8.3 The test results of this reagent are for clinical reference only and should not be used as the sole basis for clinical diagnosis and treatment. The clinical management of the patient should be considered in combination with other laboratory tests of the patient’s symptoms/signs history and treatment response.