Key Words: NewScen Morphine (MOR) Gold Rapid Screen Test
Usage/Application: Laboratory / Hospital / Pathology
Sample type: Morphine (MOR) Gold Rapid Screen Test for the qualitative detection of MOR in urine
Detection type: Qualitative
Method: Colloidal Gold Rapid Test
Function: Diagnose
Certificate: ISO9001/ ISO13485/ CE
Format: Strip, Cassette, Midstream, Uncut
Sensitivity: 100%
Specificity:100%
Total Accuracy: 100%
Reading time: 5-10 minutes
Packaging Details:
Pouch+Box+Carton packaging
(1) With our company’s Logo
(2) With the natural package
(3) With OEM package
(4) ODM
Description
1.Product Description:
NewScen Morphine (MOR) Rapid Test Kit is used for the qualitative detection of morphine with the minimum detected concentration of 300ng/mL in human urine, and for preliminary screening of morphine.
2.Principle:
NewScen Morphine (MOR) Rapid Test Kit is produced by the colloidal gold immune chromatographic method, which is to coat the corresponding drug antigen on the nitrocellulose filter membrane, and mark the specific antibody with gold colloidal particle. When testing, if there is no morphine in the sample, the gold colloid-specific antibody binds to the membrane antigen to form two red lines (one reaction line and one quality control line), proving the test result is negative. If the concentration of morphine in the sample is higher than the detection level (300ng/mL), the drug molecule will compete with the gold colloid antibody to bind, thus preventing the gold colloid specific antibody from binding to the membrane antigen, so that the corresponding red reaction line will disappear and only a red line (quality control line) will be formed in the quality control zone, proving that the test result is positive.
3.Storage:
Morphine (MOR) Rapid Test Kit should be stored at 4-30°C (do not freeze) for 24 months from the date of manufacture. Keep the test cassette in a sealed pouch until use. Once you have taken the test cassette out of the pouch, perform the test as early as possible (within 1 hour) to avoid the test cassette from becoming moist. Do not use the test beyond the indicated expiration date.
4.Sample Collection And Test Preparation:
4.1. Urine is collected in plastic cups; Urine without any preservatives.
4.2. If urine cannot be examined in time, it can be stored for 72 hours from 2℃ to 8℃.below -20℃ can be stored for a long time.
4.3. Urine avoid deterioration by multiple freeze-thaw cycles; Refrigerated or frozen urine should be restored to room temperature (18 ~ 30℃) and mixed before testing.
4.4. If the urine is turbid, centrifuge first and remove the precipitation before testing.
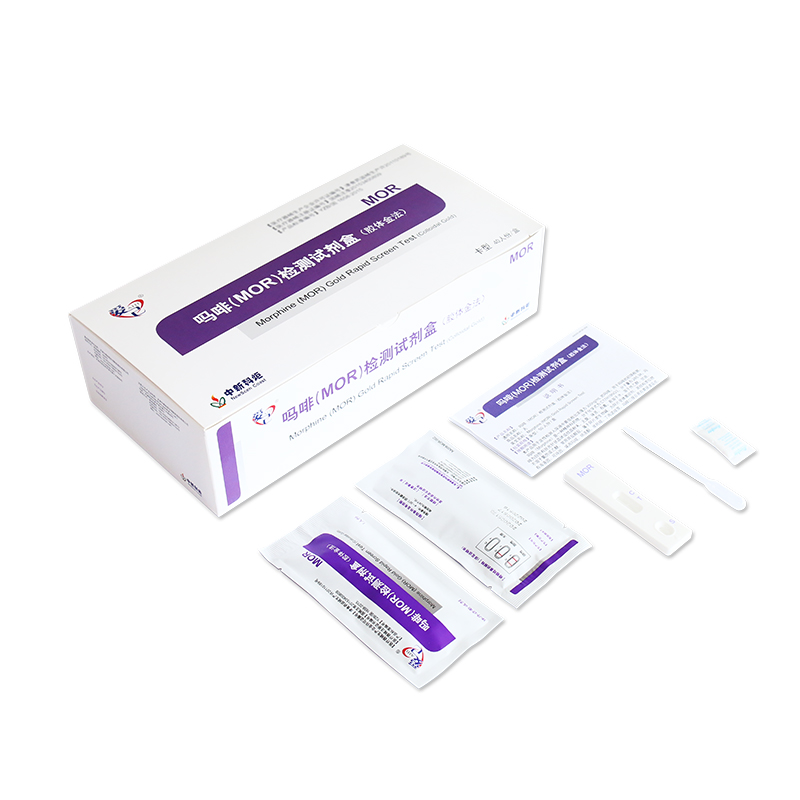
5.Procedure of NewScen Morphine (MOR) Rapid Test Kit:
5.1. Place the test cassette on a flat surface. Before unsealing the pouch, allow the test cassette to reach room temperature (18-30°C). Use it immediately once unsealed.
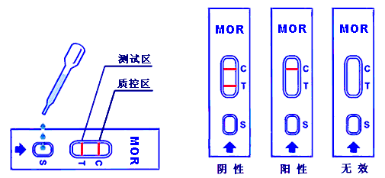
5.2. Suck urine through a plastic dropper and drop 2 to 3 drops (50 to 80µL) of urine into the sample hole (S) of the test card.
5.3. Observe the result in 10 minutes.
6.Result Interpretation:
6.1. Negative: One red line in the control zone (C) and one red line in the test zone (T). This indicates the specimen is negative.
6.2. Positive: One red line in the control zone (C) and no red line in the test zone (T). This indicates the specimen is positive.
6.3. Invalid: No red lines appear in the control zone (C), regardless of whether there is a red line in the test zone (T), indicating that the test is invalid. Discard the test cassette and perform with a new cassette.
Built-In Control
The kit has a built-in procedural control that demonstrates assay validity. A red line appeared on the control zone (C) indicates that the test runs correctly.