Key Words: Newscen HCV Antibody Rapid Test (Colloidal Gold), Rapid Diagnostic Test for Hepatitis C Virus Antibody
Usage/Application: Laboratory / Hospital / Pathology / Government Procurement / Self Testing
Sample type: Serum, Plasma or Whole Blood
Detection type: Qualitative
Method: Colloidal Gold Rapid Test
Function: Diagnose
Certificate: ISO9001/ ISO13485/ MHRA/ CE by TUV
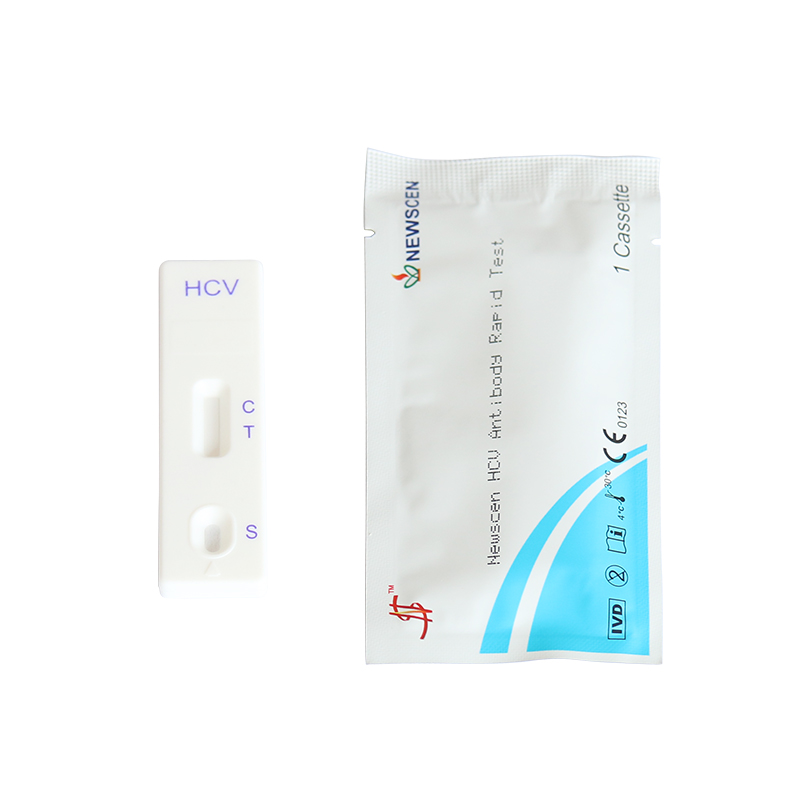
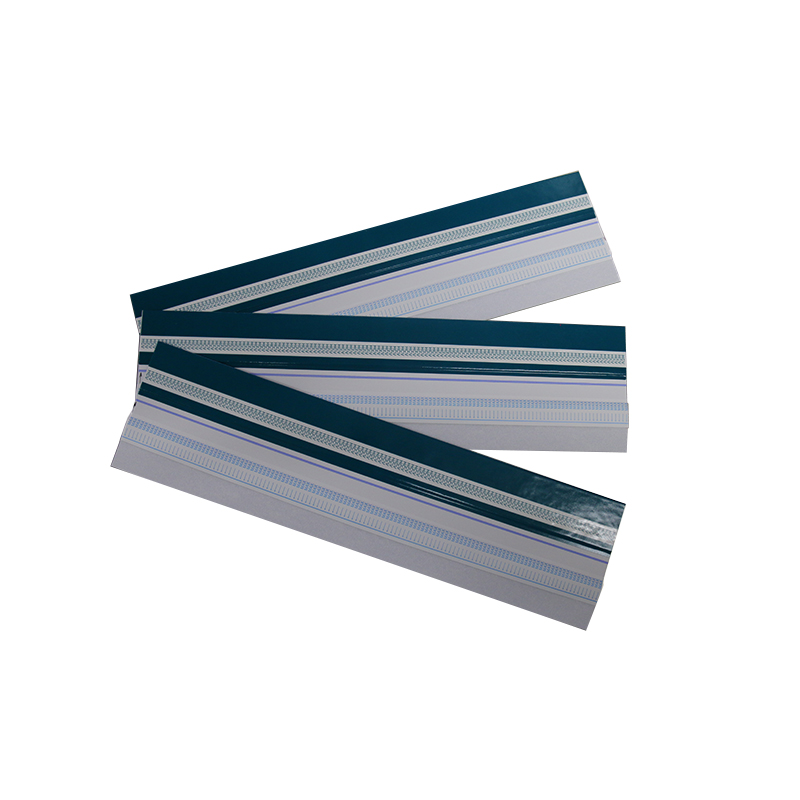
Format: Strip, Cassette, Uncut Sheet
Sensitivity: 99.76%
Specificity: 100%
Reading time: 15-30 minutes
Packaging Details:
①For Professional Use: 40 Cassette/Kit; 100 Strips/Kit; 50 Strips/Bottle (For Customization)…
② One Cassette/Box
③Uncut Sheet for OEM
Description
HCV Rapid Test Kit Manufacturer, Supplier, Exporter from China
1. Product Description:
We are counted among some of the most reliable names engaged in offering a huge gamut of HCV Rapid Test Kit. This product is extensively used for doing blood tests in patients. The given device is designed under the firm direction of the adept experts using excellent quality approved medical grade materials, sourced from the trusted experts. Our quality checkers check the product on finishing and quality standards to offer a defect-free array to the customers. Besides, the offered Blood Testing Reagent Antibody to HCV Rapid Test Device can be availed from us at market-leading rates.
Immunochromatographic test for the detection of HCV antibody in the serum or plasma.
2. Intended Use:
NewScen HCV Antibody Rapid Test is for the detection of antibodies to Hepatitis C type virus (HCV) in human serum, plasma, or whole blood collected from vein or fingertip. It is intended for use in medical institutions by trained staff.
3. NewScen Providing HCV Rapid Test With Below Specification:
4. Principle of HCV Rapid Test Kit:
HCV (Core and NS3) specific recombinant antigens are precoated onto the membrane as the capture reagent on the test zone. During the test, the specimen is allowed to react with the colloidal gold particles, which have been conjugated with HCV-specific recombinant antigens. Antibodies to HCV, if present, will specifically bind to the colloidal gold-antigen complex. When the colloidal gold-antigen-antibody complexes move to the test zone, they will specifically bind to the pre-coated antigens. At the same time, a red-colored line will develop in the test zone. The absence of this red-colored line in the test zone suggests a negative result. To serve as a procedural control, a red-colored line in the control zone will always appear regardless of the presence of antibodies to HCV.
5. Storage of HCV Rapid Test Kit:
Newscen HCV Antibody Rapid Test should be stored at 4-30°C (do not freeze) for 24 months from the date of manufacture. Keep the test cassette in a sealed pouch until use. Once you have taken the test cassette out of the pouch, it should be placed in a humidity environment of 90%RH or less, perform the test as early as possible (within 1 hour) to avoid the test cassette from becoming moist.
Do not use the test beyond the indicated expiration date. The diluent buffer should be stored at 4-30°C (do not freeze) for 24 months from the date of manufacture. After the diluent is opened, it will be stored for 24 months according to the validity period.
6. Procedure of NewScen HCV Test Kit:
6.1. Place the test cassette on a flat surface. Before unsealing the pouch, allow the test cassette to reach laboratory temperature (15-30°C). Use it immediately once unsealed.
6.2. Open the pouch and add 1 drop or 35µL of the specimen into the sample well (S).
6.3. After adding the specimen, add 1 drop or 35µL of diluent buffer vertically into the sample well (S) immediately.
6.4. Avoid dropping specimen or diluent buffer in the observation window.
6.5. Do not allow the diluent buffer bottle to touch the sample well when dropping the diluent buffer so as to prevent cross-contamination with the specimen.
6.6. Observe the result for 30 minutes once the diluent buffer is added.
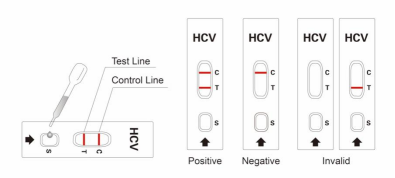
7. Result Interpretation:
7.1. Negative: No red line appear in 30 minutes in the test zone (T), only a red line in the control zone (C), which indicates that the absence of HCV antibodies or the concentration of antibodies is too low (i.e. window period of infection).
7.2. Positive: One red line in the control zone (C) and one red line in the test zone (T). This indicates the specimen contains HCV antibodies.
7.3. Invalid: If no red lines appear, the test is invalid. Discard the test cassette and perform with a new cassette.
Built-In Control
NewScen HCV Antibody Rapid Test has a built-in procedural control that demonstrates assay validity. A red line appeared on the control zone (C) indicates that the test runs correctly.
8. Limitation:
8.1. NewScen HCV Antibody Rapid Test must be used in accordance with this instruction for use to obtain an accurate result.
8.2.The positive results using the Newscen HCV Antibody Rapid Test suggests the presence of HCV antibodies in the specimen, and the intensity of the test line does not necessarily correlate with the HCV antibody titer in the specimen. The Newscen HCV Antibody Rapid Test is intended as an aid in the diagnosis of HCV infection.
8.3.The negative results do not exclude the possibility of exposure to HCV or infection with HCV. Antibody response to recent exposure may take several months to reach detectable levels.
8.4. A person who has HCV antibodies is presumed to be infected with the virus. Additional testing and medical evaluation are required to determine the state or associated disease.
9. International Certificates Of Newscen HCV Test Kits:
*CE (List A)
NewScen patented HIV and HCV RDTs have obtained CE approval by TUV laboratory evaluation, hot sold domestically and abroad by receiving high concentration by customers.
*MHRA
Newscen HCV Antibody Rapid Test has been granted regulatory approval by the Medicines and Healthcare products Regulatory Agency (MHRA).