Exhibition Overview
From 17–20 November 2025, the MEDICA International Medical Trade Fair will once again take place in Düsseldorf, Germany under the theme “Meet Health, Future, People.”
The exhibition continues to serve as a global platform for medical technology innovation, highlighting future-oriented healthcare solutions and industry development trends.
Established in 1969, MEDICA is widely recognized as the world’s No. 1 comprehensive medical trade fair, bringing together over 5,000 exhibitors from 70+ countries and attracting 80,000+ professional visitors annually. Its scale, professionalism, and international influence are unmatched within the global IVD and medical device sectors.
NewScen’s Highlight Showcase at MEDICA 2025
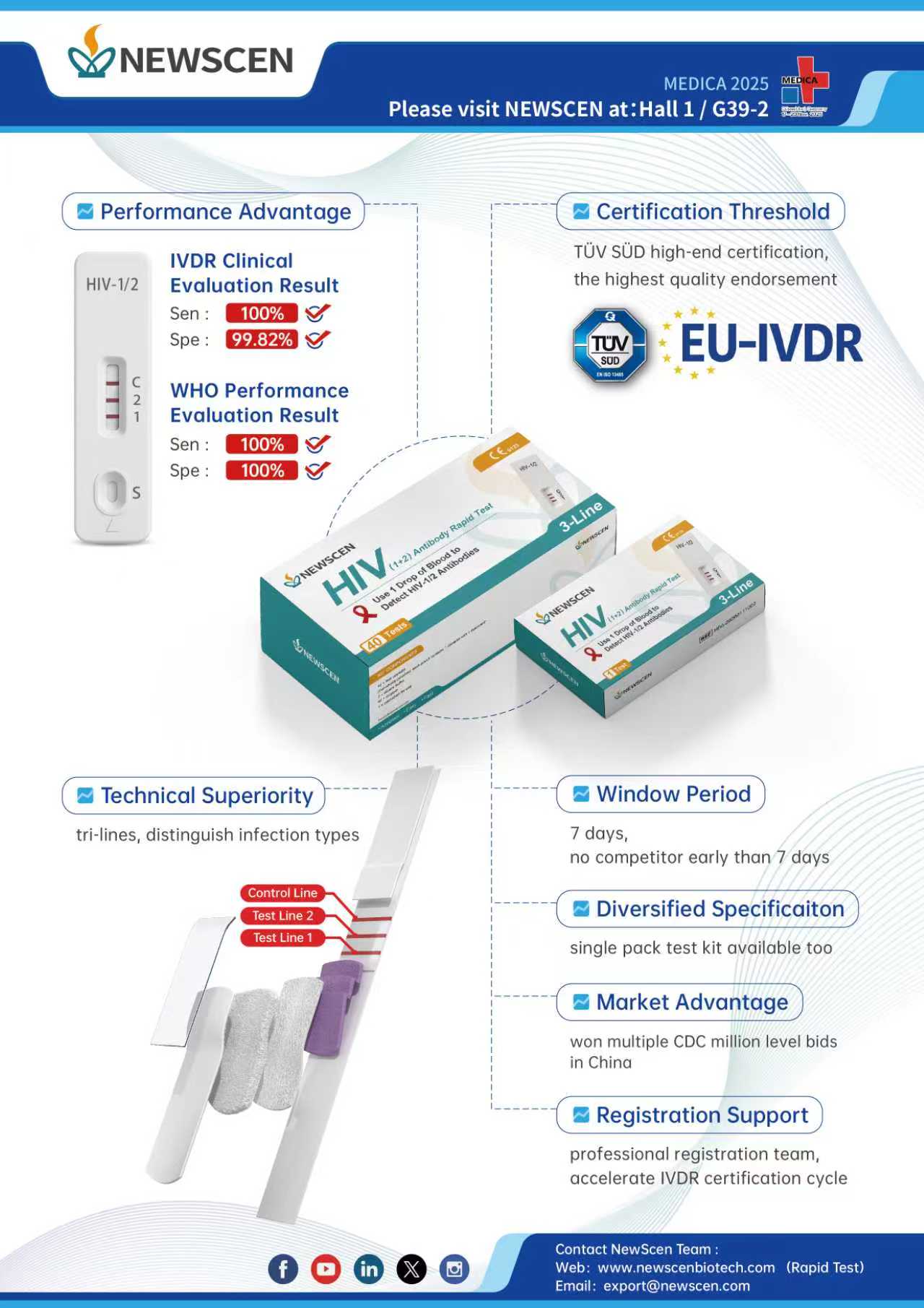
At MEDICA 2025, NewScen Coast Bio-Pharmaceutical (NewScen Biotech) will make a strong appearance with its newly IVDR-certified HIV rapid diagnostic test, alongside a full portfolio of infectious disease rapid tests and home-use self-testing products, including HCV, SF (Serum Ferritin), FOB, HCG, LH, FSH, and more.
Our exhibited portfolio reflects NewScen’s continued commitment to high-quality rapid diagnostics, addressing both professional clinical screening and consumer self-testing demand worldwide.
IVDR-Certified HIV (1+2) Tri-Line Rapid Test
Higher Analytical Accuracy | Enhanced Stability | Reliable Performance
The HIV (1+2) Antibody Rapid Test is the result of NewScen’s long-term R&D investment and stringent quality control.
The product has successfully obtained EU IVDR Class D CE certification (Notified Body: TÜV SÜD), representing the highest regulatory standard for infectious disease rapid tests under IVDR.
Designed for professional use, the assay delivers:
-
High analytical sensitivity & specificity
-
Clear tri-line interpretation
-
Short turnaround time
-
Broad specimen compatibility (whole blood—including fingerstick—serum, plasma)
It provides an advanced HIV primary screening solution for laboratories, hospitals, NGOs, public health programs, and point-of-care testing (POCT) settings.
Comprehensive Portfolio of Rapid Testing & Self-Testing Products
NewScen will also showcase multiple key products at MEDICA, including:
Infectious Disease Rapid Tests
-
HCV Rapid Test
(CE IVDD List A; IVDR Class D application in progress)
Home-Use Self-Testing Series
-
SF (Serum Ferritin) Test – Finger-prick blood
-
FOB (Fecal Occult Blood) Test
-
HCG Pregnancy Test
-
LH Ovulation Test
-
FSH Menopause Self-Test
Our portfolio covers:
-
Clinical primary screening
-
Community / POCT scenarios
-
Consumer home-use health monitoring
Providing complete, multi-scenario rapid diagnostic solutions for global distributors and healthcare providers.
Visit NewScen at MEDICA 2025
We sincerely invite you to visit NewScen’s booth at MEDICA 2025.
Our team will present our IVDR-certified innovations, core POCT platforms, and global partnership models, supporting you in expanding business opportunities in Europe and other regulated markets.
Together, let’s advance accessible, reliable, and high-performance rapid diagnostics worldwide.
📧 Contact us today:
Email: export@newscen.com