The HIV (1+2) Antibody Rapid Test by NEWSCEN (NewScen Coast Bio-Pharmaceutical Co., Ltd.) is expected to obtain the IVDR CE certification (Regulation EU 2017/746) officially in Q3 of 2025, issued by TÜV SÜD Product Service GmbH, a globally renowned third-party endorsement organization of the EU, in accordance with IVDR regulations. It would be a significant milestone for NEWSCEN’s internationalization strategy by obtaining the IVDR CE certificate of Class D high-risk rapid diagnostic product, the access to EU and other highly regulated market, of which would enable us to better serve the global IVD market. The upcoming IVDR CE certification has fully demonstrated NEWSCEN’s professional strength in aspects of product research and development, quality management system and capabilities in compliance with globalized regulations.
It would be a significant milestone for NEWSCEN’s internationalization strategy by obtaining the IVDR CE certificate of Class D high-risk rapid diagnostic product, the access to EU and other highly regulated market, of which would enable us to better serve the global IVD market. The upcoming IVDR CE certification has fully demonstrated NEWSCEN’s professional strength in aspects of product research and development, quality management system and capabilities in compliance with globalized regulations.
Core Advantages of HIV (1+2) Antibody Rapid Test by NEWSCEN
✅ Triple-Lines Design, Clearer Distinction of HIV Infection Type
Simultaneous detection and independent indication of HIV-1 and/or HIV-2 antibodies presence. The NEWSCEN patented triple-lines design HIV rapid test would greatly assist in the rapid and accurate differentiation of HIV infection type. Makes it an ideal choice for clinical use and suitable for various high-demanding diagnostic scenarios.
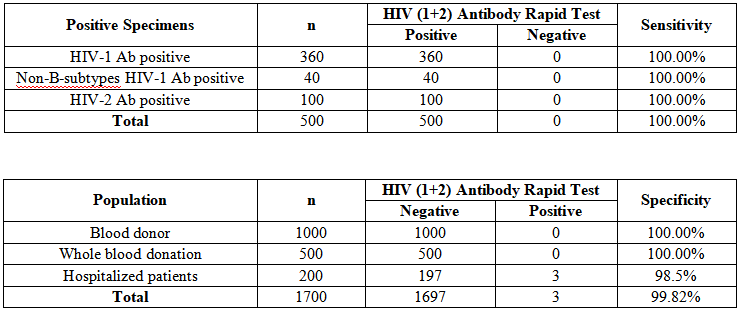
✅ High Sensitivity & Specificity
Clinical performance validated by multiple famous clinical centers and institutions. Simple and convenient operation, accurate and reliable test result. Time to result in just 15 minutes. Proven to be applicable for large scale testing scenarios.
Clinical Performance:
Sensitivity:100.00%
Specificity:99.82%
✅ Full Regulatory Compliance, Comprehensive Certification
Fully aligned with IVDR requirements and about to be officially certified by TÜV SÜD soon. Management and operation in strict accordance with the ISO 13485 Quality Management System. Comprehensive Master File and Complete Technical Documents, clear and traceable registration path.
✅ Multiple Packaging Formats, Satisfy Diversified Applications
NEWSCEN provides a range of packaging formats of the HIV rapid test (IFU in different language versions optional), applicable and suitable for various end-users and markets including hospitals, labs, CDC, Public Health Project, etc.
Pack Size:1 T/Box, 2 T/Box, 5 T/Box, 25 T/Box, 40 T/Box
Specimen:Fingertip whole blood, venous whole blood, serum, plasma.
✅ Upgraded Packaging Recognition for Global Markets
Enhanced and upgraded international labeling and packaging design improve the brand recognition and market adaptability to meet diversified global needs.
🌍Flexible, Compatible and Applicable for Global Markets
HIV (1+2) Antibody Rapid Test by NEWSCEN has demonstrated the vigorous adaptability for the global market and dynamic suitability to satisfy diverse healthcare needs since launched in 2002, by the satisfactory performances in the hospital laboratories, clinical diagnostic institutions, government procurement programs, NGO partnership cooperations, international bidding projects and commercial distributions worldwide. Market driven, technology empowered, based on professional and efficient diagnosis solution and considerate one-stop service, NEWSCEN would focus on providing our clients with market oriented and tailored services to meet the local healthcare expectations, and offering flexible collaboration models either in highly regulated regions or fast-moving commercial channels.
🤝 Opportunities — Global Partnerships and Cooperations
NEWSCEN has always been dedicated to the life-long pursuing career of human health since established and we sincerely welcome the most esteemed distributors, agents, medical institutions and government procurement partners globally to request samples for evaluations, initiate registration qualification reviews and explore cooperation opportunities. We are more than pleased to work with you together for the business development globally with professional HIV diagnosis solutions, and make continuous contributions to the career of human health.
📧 Contact us today:
Email: export@newscen.com